Recently, a parent sought help from us regarding a question from her daughter’s homework in our Parent Support Group on Facebook.
Read Also
Question
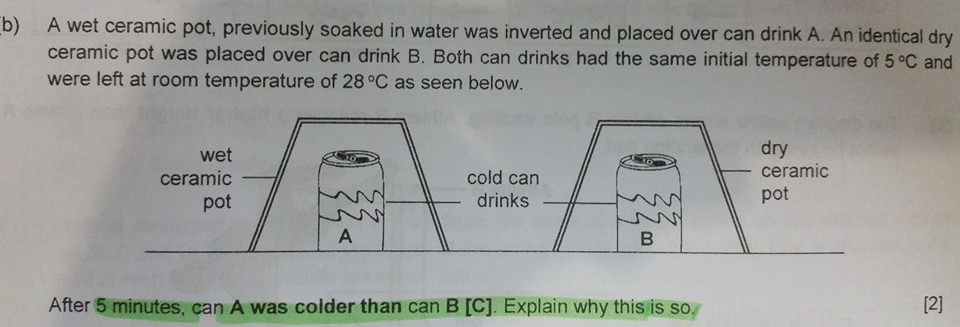
A wet ceramic pot, previously soaked in water was inverted and placed over drink A. An identical dry ceramic pot was placed over Drink B. Both can drinks had the same initial temperature of 5°C and were left at room temperature of 28°C as seen below.
After 5 minutes, Can A was colder than Can B. Explain why is this so.
Thought Process
When we approach this question, we need to focus on the changed variable, which is the ceramic pot, followed by the transfer of heat energy between the can and the air inside the respective pots.
Answer
The water in the wet ceramic pot gained heat from the air in Pot A to evaporate, causing the air in Pot A to become cooler than the air in pot B.
Thus, Can A will gain heat slower from the cooler air inside Pot A, causing Can A to be cooler than Can B after 5 minutes.
I hope that this article helps to show you and your child the thought process behind how to approach such questions testing on the topic of Heat Energy.
If you like our methodology, we’ve some upcoming workshops:







