Welcome back to the Matter Series! One reason why students fail to tackle examination questions effectively and accurately is due to their inability to integrate concepts from different topics together.
In today’s article, I will attempt to bridge the gap between the concepts from the topics of Body Systems and Matter.
Read Also
Many students tend to regard the movement of air in our lungs to be under the topic of Body Systems. As a result, they are often unable to link the concept of the movement of air in the lungs to the properties of matter.
Let us first look at the diagram for the model of a respiratory system shown below.
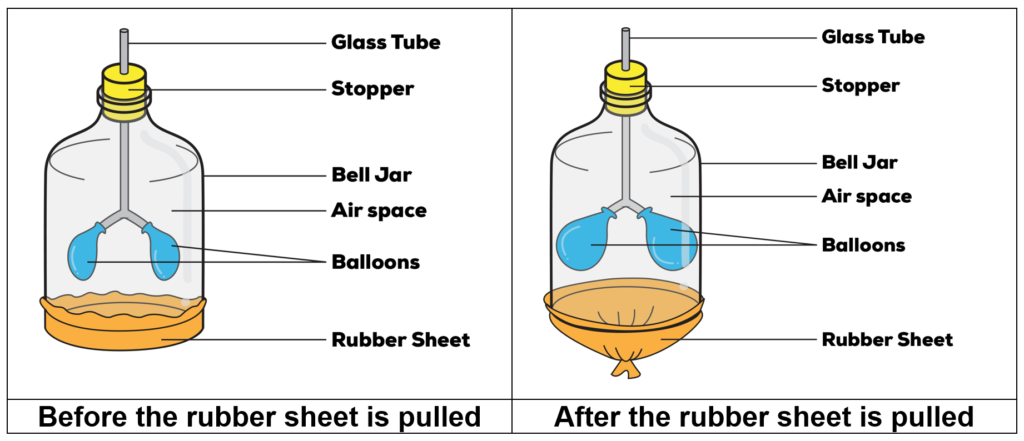
Integrating Matter with Body Systems- Movement of Air in Our Lungs – Rubber Sheet Comparison
When the rubber sheet is pulled, the volume of the bell jar increases. Thus, there would be additional space inside the bell jar. The air outside the bell jar (surrounding air) would then enter the bell jar through the glass tube to occupy the additional space inside the bell jar. As the glass tube is connected to the balloons, air enters the balloons, causing them to inflate.
Now, using the properties of matter that we have learned, what can we conclude about the mass and the volume of air inside the balloons?
Here Are Some Guiding Questions
- Does the mass of air in the balloons change?
- Does the volume of air in the balloons change?
Q1: Does the mass of air in the balloon change?
A1: Yes, there would be an increase in the mass of the balloon. When air enters the balloons, there would be MORE AIR inside the balloons, causing its mass to increase.
Q2: Does the volume of air in the balloons change?
A2: Yes. Since the balloon inflates and increases in size, this shows that there is more air inside the balloon. Thus, the volume of air inside the balloon increases and occupies more space.
Sounds easy? Can you now think of a situation where there is an increase in the volume of the balloon and no increase in the mass of the balloon?
If you are thinking about the placing a balloon in hot water, then you are absolutely right!

Placing a tied balloon in hot water would cause the air inside the balloon to gain heat from the warmer water to expand and increase in volume. This causes the balloon to inflate. However, since there is no additional air entering or escaping from the balloon, the mass of the air in the balloon remains the same.
Remember: The volume of air in the balloon does not always affect the mass of air in the balloon.
You might be wondering now…how is this possible?
To understand this concept better, I would like you to visualise air in terms of small balls.

When air expands, the small balls move apart from each other but the total number of balls remains the same.
Now with that understanding of matter, I would like to challenge you with this question here.
Let’s Take A Look At This Question

Source: Methodist Girls’ School – 2017 P6 Preliminary Science Examination Paper [Q16]
Let us analyse this question together:
“After 15 minutes, both the mass and the volume of air in the balloon in each set-up increased”
In order for the mass of the balloon to increase, there is only one possible reason: Air must have entered the balloon.
There are two possible reasons why there is an increase in the volume of air:
Reason 1: There is more air in the balloon. Thus, more air takes up more space in the balloon, causing its volume to increase.
Reason 2: There is no change in the number of air particles in the balloon. However, there is an expansion of air in the balloon, causing the air to take up more space in the balloon and increase in volume.
Let Us Analyse The 4 Set-ups
In Set-up A

Since the balloon is tied, no air can enter or escape the balloon. Thus the mass of the air in the balloon remains the same.
As the balloon is submerged in hot water, the air in the balloon would gain heat from the water at 100°C to expand and increase in size.
The increase in size shows that the air in the balloon has a larger volume.
Here’re The Outcomes:
- No change in the mass of the air in the balloon
- Increase in volume of air in the balloon
In Set-up B

The air in the bottle would gain heat from the water at 100°C to expand and rise up into the balloon, causing it to inflate and increase in size.
The balloon would then have more air in it, increasing the mass of air in the balloon. The balloon’s larger size would also mean that the air in it has a larger volume.
Here’re The Outcomes:
- Increase in the mass of air in the balloon
- Increase in the volume of air in the balloon
In Set-up C

The air in the bottle would lose heat to the ice and contract, causing the air to decrease in volume. Thus, there would be more space in the bottle, causing the air from the surroundings to enter the bottle to occupy the extra space in it.
Since the balloon is wrapped around the mouth of the bottle, the air that enters the bottle goes into the balloon, causing it to inflate and increase in size.
Thus, the balloon would have more air in it, increasing the mass of air in the balloon. The balloon’s larger size would also mean that the air in it has a larger volume.
Here’re The Outcomes:
- Increase in the mass of air in the balloon
- Increase in the volume of air in the balloon
In Set-up D

The air in the bottle gains heat from the water at 100°C to expand and increase in volume. This exerts a force on the balloon, causing the air in the balloon escape. Thus, the balloon deflates and decreases in size.
The balloon would then have less air in it, decreasing the mass of air in the balloon. The balloon’s smaller size would also mean that air in it has a smaller volume.
Here’s The Outcomes:
- Decrease in the mass of air in the balloon
- Decrease in the volume of air in the balloon
As such, the answer for the question would be Option 2 – B and C only.
In Conclusion
I hope this article has provided you with a much better understanding of how to analyse Matter related questions.
Stay tuned for more insightful content from us!
If you like our methodology, please click here to explore our Matter Techniques™ Masterclass for P5 & P6 students.








