Introduction
Do you find drawing conclusions from the results of a chromatogram challenging?
In this Chemistry blog post, I will draw similarities between paper chromatography and a track race to help you understand key concepts of paper chromatography better.
You can also watch our explainer video on The Pique Lab’s YouTube channel for free!
Read Also:
Let’s Take A Look At This Chemistry Question
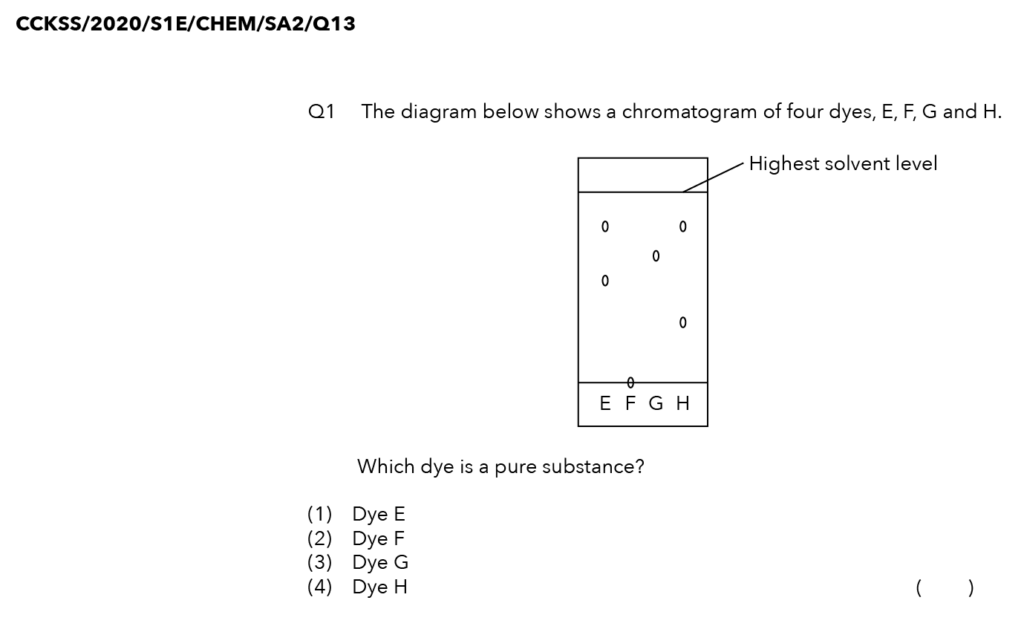
Source: Chua Chu Kang Secondary School – 2020 Chemistry S1E SA2 Examination Paper [Q13]
Let us begin by defining a chromatogram.
🔎 What Is A Chromatogram? 🔎
A chromatogram is the result of a paper chromatography — a separation technique used to separate and identify small amounts of substances that are dissolved in a solvent. We can also use it to test the purity of a substance.
Let us look at the results of the chromatography.
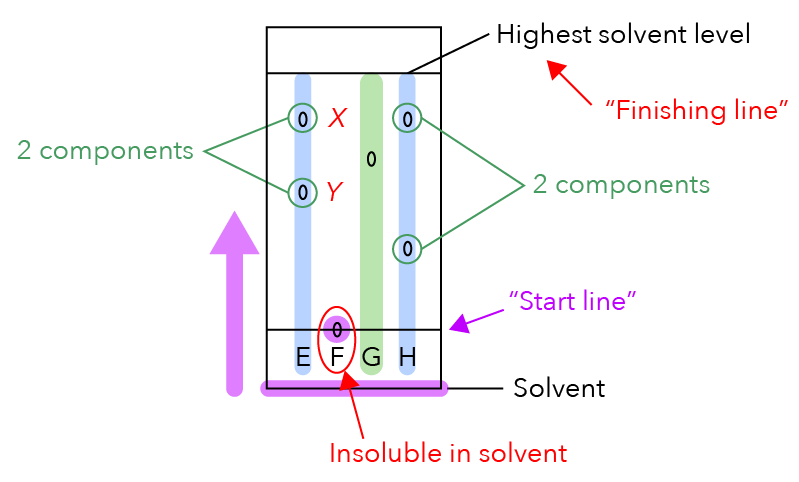
What is the highest solvent level labelled in the diagram below?
If we were to compare it with a race, the highest solvent level is like the finishing line.
Meanwhile, the part I labelled in purple is the start line of the race.
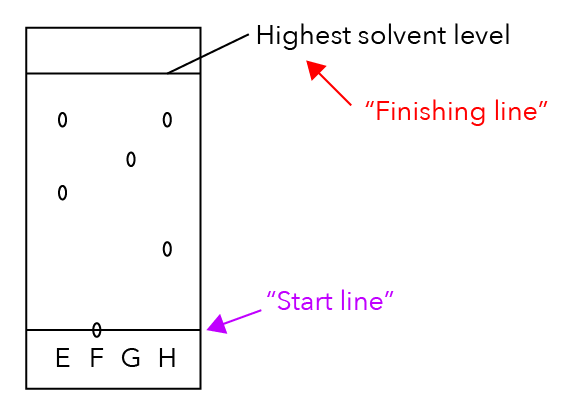
The start line is where I will put a tiny drop of the sample, just like what you see in F.
This chromatography paper is dipped into the solvent and the solvent moves up the paper, just like how racers run towards the finishing line.
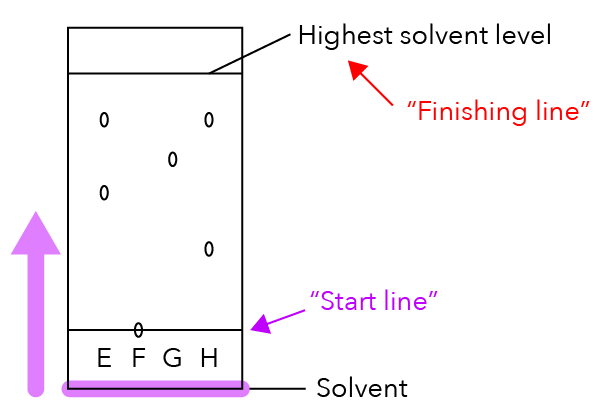
If the sample is made up of two or more components, the different components will move at different speeds together with the solvent and they will start to separate.
Let’s take a look at the results.
Let’s Analyse Dye E
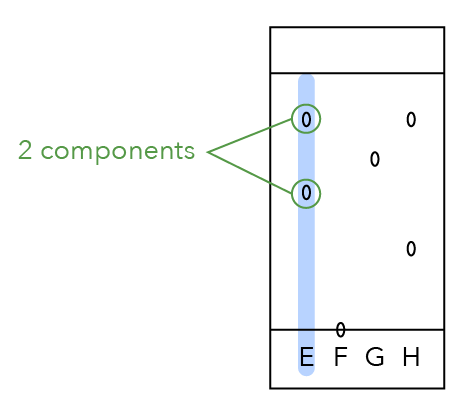
How many spots do you see for E?
There are two spots! This means that E is made up of two components, which are separated to form two visible dots on E.
Let us compare the two dots. What can we say about spot X below? It is closer to the finishing line.
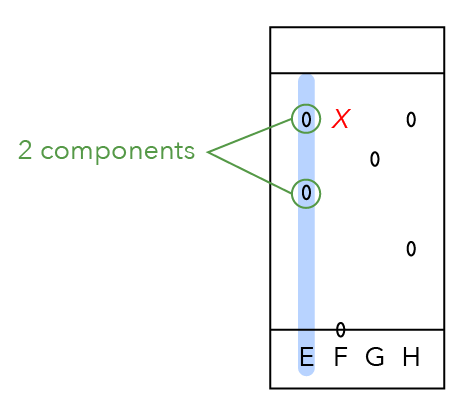
Do you think it moved faster or slower?
Since it is closer to the finishing line, it means that it moved faster and its component is more soluble in the solvent.
Let us take a look at spot Y, which is further away from the finishing line.
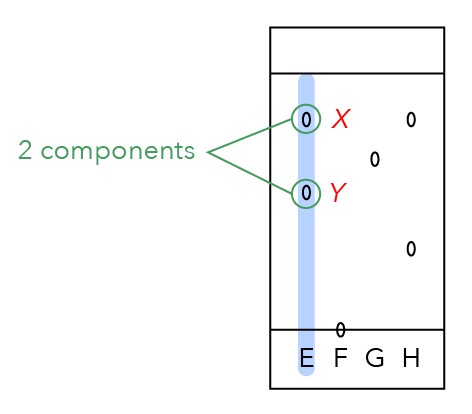
Is spot Y more soluble or less soluble?
Since it is further away from the finishing line, it has moved slower in the solvent and is less soluble in the solvent.
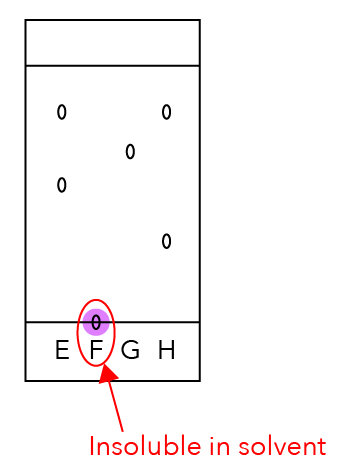
Let’s Analyse Dye F
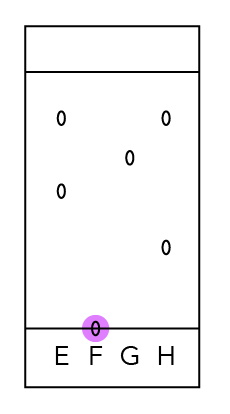
Dye F has only one dot.
Does it mean that it is only made up of one component? No!
You’ll notice that the dot is still on the start line and has not moved up together with the solvent.
We can compare it with a runner who did not run the race and just stayed at the start line.
Since F did not move with the solvent, it is insoluble in the solvent.
Hence, giving no results.
Let’s Analyse Dye G
How many components is dye G made of?
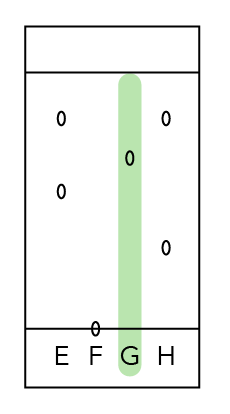
Since we can only see one dot, this means that it is made up of only one component and is not mixed with any other substance.
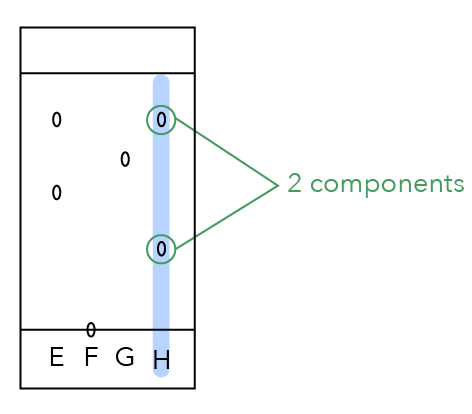
Let’s Analyse Dye H
Like dye E, dye H is also made up of two components since we can see two dots.
Let us label them!
Thought Process
Now that we’ve analysed the results of the chromatogram, let’s review the question.
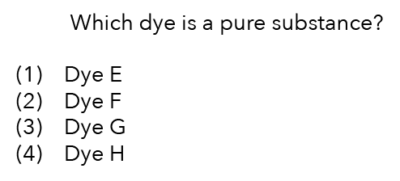
Source: Chua Chu Kang Secondary School – 2020 Chemistry S1E SA2 Examination Paper [Q13]
We are looking for the dye, which is a pure substance.
🤔 What Is A Pure Substance? 🤔
A pure substance is made up of only one component and cannot be mixed with other substances.
Let’s take a look at our chromatogram.
How many dots should we look for in the chromatogram?
We should look for the chromatogram with only one dot that has moved up the solvent.
Which result shows us that there is only one component in the dye?
It is dye G!
Answer For Q13
Option (3)
Conclusion
I hope that after reading this Chemistry blog post, you will remember the following key points when analysing a chromatogram:
- A single dot on the chromatography paper means the substance is made up of only one component.
- If the sample is made up of two or more components, the different components will move at different speeds together with the solvent and they will start to separate — resulting in two or more separate dots.
- When a dot does not move from the start line, it means that it is insoluble in the solvent.
Stay tuned for more Secondary 1 Chemistry blog posts!
If you like our methodology, we’ve some upcoming workshops: