Introduction
Have you tried dissolving Milo powder or sugar in water? Was it easier to dissolve them using hot water or cold water?
It is easier to dissolve them using hot water, right?
In this Chemistry blog post, we will be discussing why solids dissolve faster in hot water than in cold water by analysing a question from the 2017 Zhonghua Secondary School (ZHSS) S1E SA2 Examination Paper on the topic of Solutions and Suspensions.
You can also watch our explainer video on The Pique Lab’s YouTube channel for free, by clicking the link.
Read Also:
Let’s Take A Look At This Chemistry Question
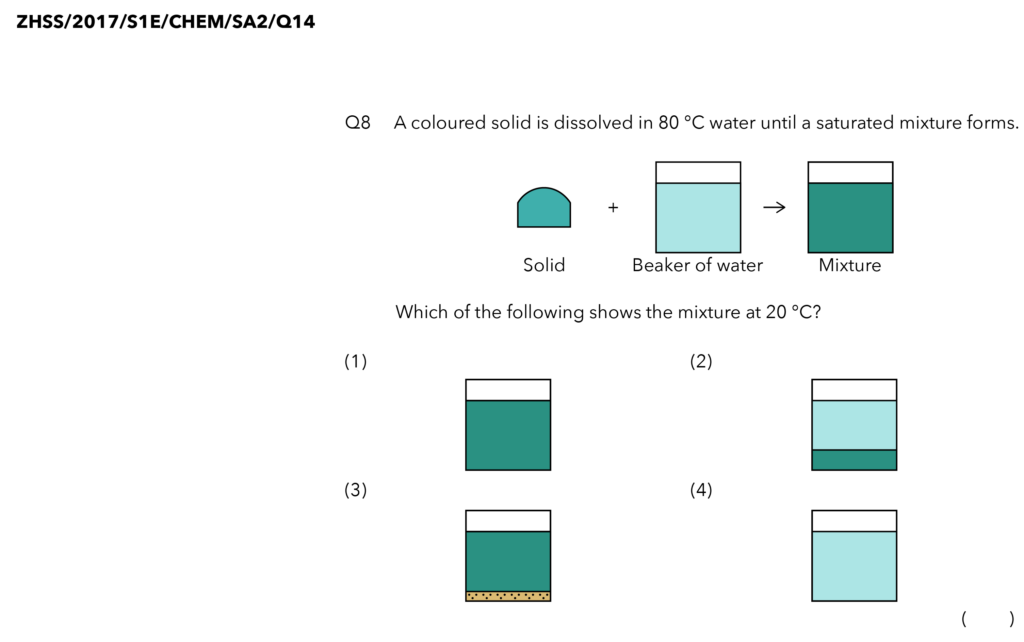
Source: Zhonghua Secondary School – 2017 S1E SA2 Examination Paper [Q14]
Thought Process
🔥 Why Is It Easier To Dissolve Solids In Hot Water? 🔥
The solubility of solids increases as the temperature of the water increases. Therefore, more solids will dissolve in hot water.
Let’s look at the diagram below.
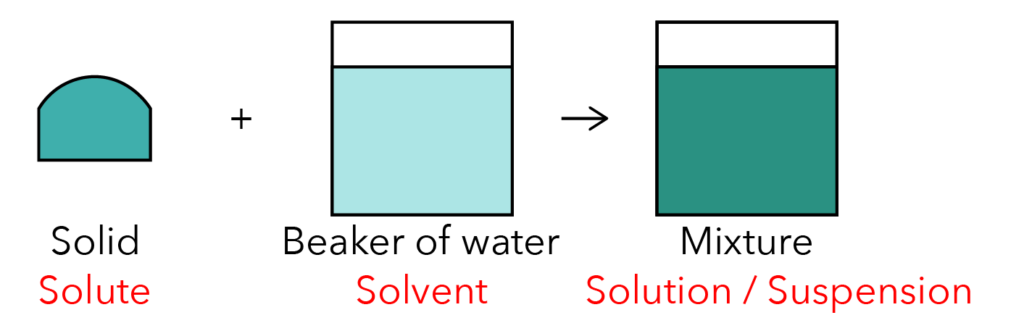 Source: Zhonghua Secondary School – 2017 S1E SA2 Examination Paper [Q14]
Source: Zhonghua Secondary School – 2017 S1E SA2 Examination Paper [Q14]
The solid is also known as the solute. The beaker of water is called the solvent. The resultant mixture formed can either be a solution or a suspension.
When the solids dissolve into the water, will there be any undissolved solids left in the mixture?
No, the mixture will look the same or homogenous. We call this a solution.
Let me use this analogy: Let us say that the students are the solids and the classroom is the water.
When all solids dissolve in water to form the solution, that is similar to when students enter the classroom. We would not see them outside along the corridors anymore.
Similarly, we won’t see any undissolved solids in our beaker of water.

The question says a saturated mixture has formed.
🥛 What Is A Saturated Solution? 🥛
It means that the maximum amount of solid has dissolved in the water.
Going back to our analogy, if the classroom can hold a maximum of 40 students, this means that exactly 40 students have entered the class.
What happens if we try to squeeze more students into the classroom?
Will they be able to get in? No, they won’t get in and we will see them hanging outside along the corridors.

Similarly, if I have a saturated solution, can I dissolve any more solids into my saturated solution?
No. The extra solids can’t dissolve and we will see the undissolved solids settling at the bottom, just like the extra students waiting along the corridors.
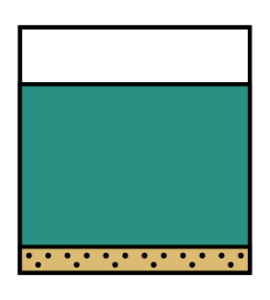
Let us go back to the question. What happens if we take the saturated solution that was at 80°C and was cooled down to 20°C?
Remember that substances contract at a cooler temperature.
Going back to our analogy of the classroom — Imagine the classroom contracting now. Will it hold more or less students?

It will hold fewer students because there is less space. Similarly, at lower temperatures, less solids can dissolve in the solution.
Remember that the solubility of the solids decreases at lower temperatures.
Imagine that the classroom that can originally hold a maximum of 40 students now has shrunk and can only hold a maximum of 10 students. What will happen to the other students?
They will have to go outside along the corridors.

Similarly, when you cool down a saturated solution, the cool solution cannot hold as much solute compared to when it is hot.
So the excess solute will be kicked out of the solution and start to form crystals, which will settle to the bottom of the beaker.
Now, we are looking for some solids at the bottom of a beaker of coloured solution.
Let’s Analyse Option (1)

Does option (1) show any solid settling at the bottom of our beaker?
No. It just shows a beaker of coloured solution. So, this is not the correct option.
Let’s Analyse Option (2)
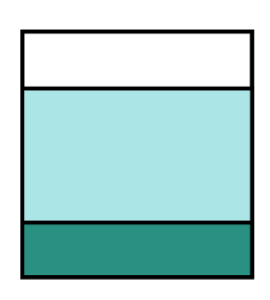
Option (2) shows some solids at the bottom of the beaker of a solution.
But if you look carefully, it shows all the solids at the bottom of the beaker. None of them dissolved in the water at all.
Using our analogy, this is like all 40 students got kicked out of the classroom. But we should have at least 10 students in the classroom. This eliminates option (2).
Let’s Analyse Option (3)
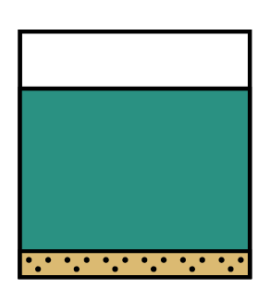
Does option (3) show some solids at the bottom of a coloured solution? Yes, it does!
This is in fact our correct answer but let’s just take a look at the final option to understand why it is not the correct answer.
Let’s Analyse Option (4)
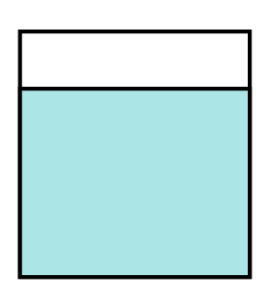
This is just a beaker of water. Where are all the solids? The solids have disappeared.
Remember our analogy? 30 students are kicked out of the classroom to the corridors. I should still be able to see those students. They didn’t disappear.
So option (4) shows no solids at all and the solids have disappeared. This cannot be our answer, and this confirms our answer as option number (3).
Suggested Answer

Option (3).
Conclusion
I hope that our analogy of the classroom helped you understand how changing temperature affects the mixture.
Remember that substances contract at a cooler temperature.
Therefore, fewer students will fit inside a classroom once it shrinks, the same way that fewer solids can dissolve in lower temperatures.
Keep a lookout for more Secondary 1 Chemistry blog posts on the topic of Solutions & Suspensions.
If you like our methodology, we’ve some upcoming workshops: